Ever heard of Lipid based nano-particles? Well, these are simply microscopic drug vehicles through which drugs are delivered to specific tissues. The major aim of solid lipid nanoparticles (SLN) in terms of drug delivery is to reinforce the bioavailability and efficacy of medicine, and control the non-specific toxicity, immunogenicity, pharmacokinetics and pharmacodynamics of drugs.
There are five categories of lipid based nanoparticles on the basis of their physicochemical properties and method of fabrication. These categories are niosomes, liposomes, solid lipid nanoparticles, transferosomes and nanostructured lipid carriers.
Liposomes protect drugs efficiently. They are highly target-specific but they have certain limitations in dermal delivery because they can’t penetrate in to the stratum corneum, moreover they can’t encapsulate hydrophilic drugs.
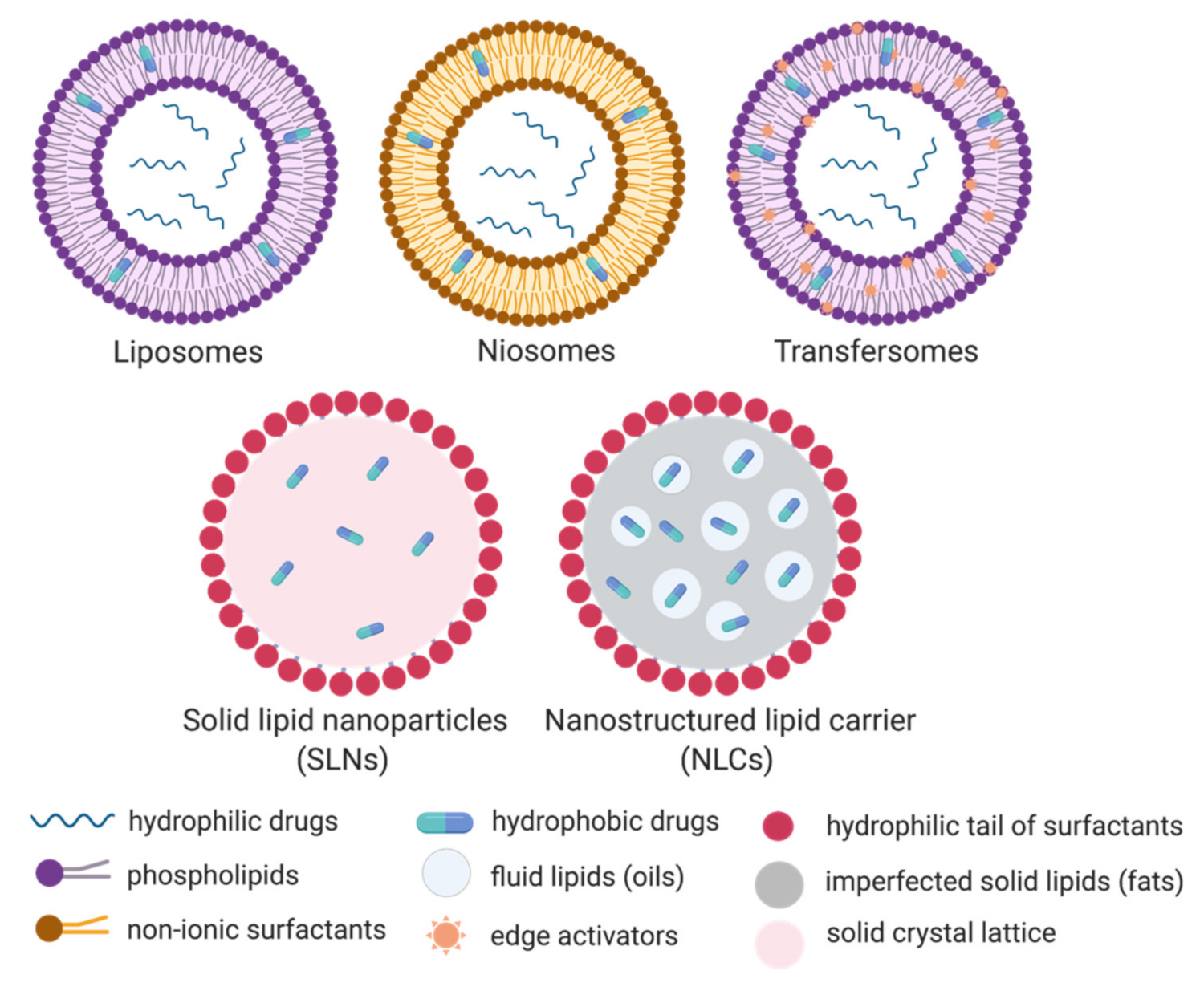
While niosomes are good alternatives of Liposomes. They are composed of cholesterol and non-ionic surfactants in aqueous media. Although they have good properties but they still failed to achieve FDA approvals for drug delivery due to leakage of drug and aggregation of particles.
Transferosomes are composed of cholesterol, edge activators and phospholipids. They exhibit efficient entrapment and penetration power, but they are costly. These differing types of nanoparticles are often wont to encapsulate both hydrophobic and hydrophilic drugs and act as an efficient drug carrier.
Drug Delivery by Lipid Based Nanoparticles:
There are wide selection of clinical applications of liposomes. Twenty-one products based on liposome have been approved for clinical use. These include one of the well-known drug Doxil. FDA has approved many other effective nano-drugs such as Depocyt, Lipodox, AmBisome, Inflexal V etc. Most of these liposome based drugs are used in oncology but they also have applications in treating fungal infections and pain relief.
Lipodox, Myocet, Doxil and liposomal doxorubicin are used to breast cancer. Amphotericin B is one such drug used for the treatment of fungal infections.
Marqibo that was initially used for treating leukemia in 2009 was approved for treatment of solid tumour in 2012. Many liposomes are also being used in chemotherapy. There are multiple liposomes that are under clinical trials for his or her use in cancer treatment.
Gene Therapy by Lipid Based nanoparticles:
Nowadays nucleic acid based therapeutics is blossoming and have great potential for treating wide range of disorders. These include messenger RNA (mRNA), small activating RNA (saRNA) and small interfering RNA (siRNA). But these nucleic acids confront immediate degradation by endonucleases. Lipid nanoparticles or liposomes act as shield for them and increase their efficiency of targeted delivery and also prevent degradation. Examples of the success of gene therapy by using lipid based nanoparticle includes Onpattro and GIVLAARI, both of them have been approved by FDA.
There is a pharmaceutical company called Dicerna Pharmaceuticals in USA that mainly focuses on RNA based medicines. It has formulated a double stranded RNA that targets Oncogene MYC which leads to suppression of cancer but its clinical studies has been stopped because the results didn’t meet the expectations.
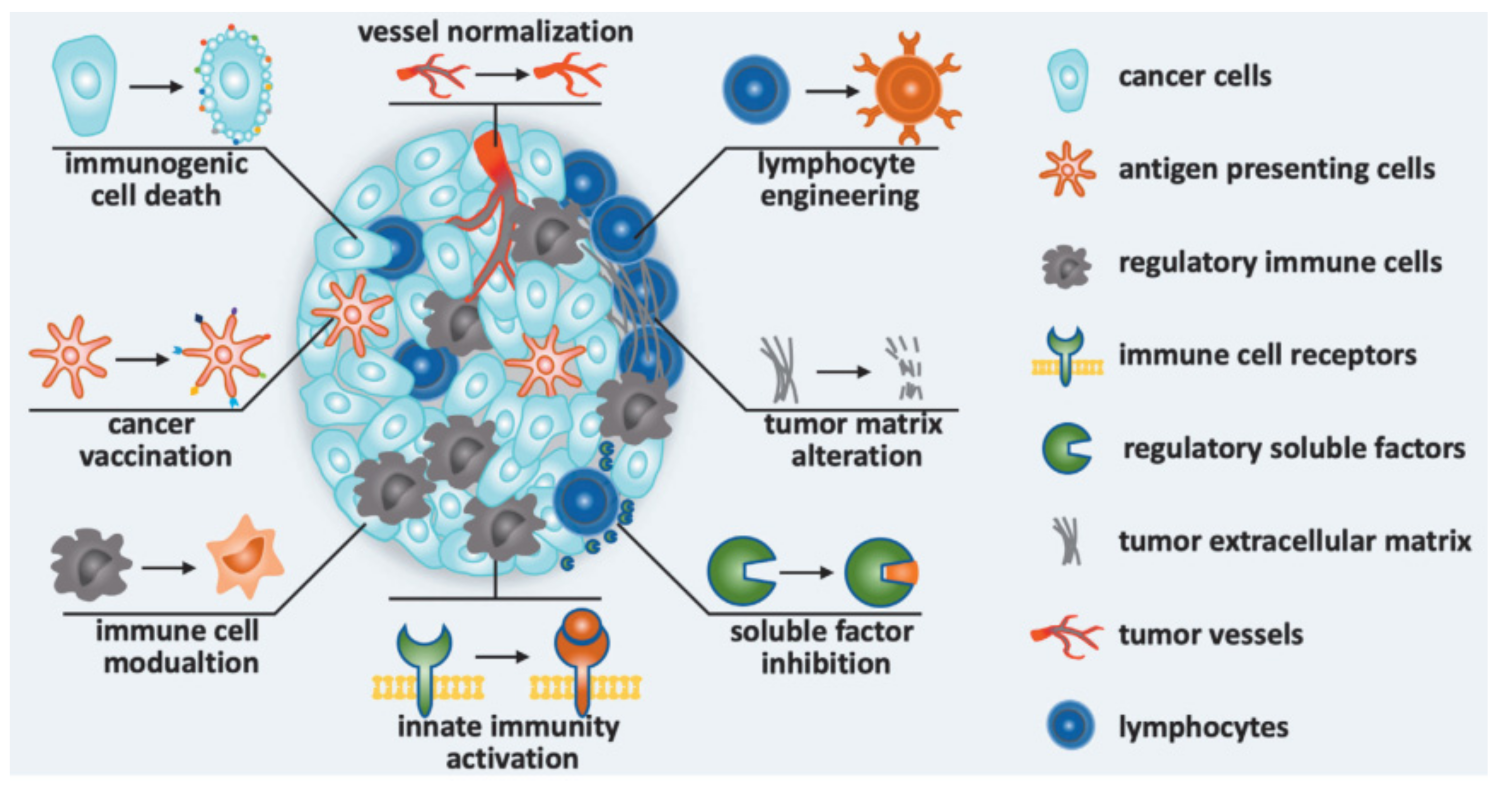
]. Image: MDPI
Another drug has been developed by Wagner et al. that’s a nano liposome EphA2 siRNA that exhibits neutral charge and reduces toxicity of the organ. EphA2 is overexpressed in cancerous cells. This Nano liposome interferes with the proliferation of cancer cells and decreases the growth of tumor. It has showed effective results. Now it is under phase 1 clinical trials where it will be used for solid tumors.
COVID-19 vaccine and Lipid Based Nanoparticles:
Whole world has become a victim of a deadly virus that initially emerged in Wuhan city, Hubei province of China by the end of 2019. Throughout the globe scientists worked hard to understand the nature, properties, preventive measures and treatment of this deadly virus. It is spreading in uncontrolled manner and it is the need of the hour to formulate vaccines along with the treatment of infected persons.
Vaccines based on nucleic acids for COVID-19 has gained much attention and now are under clinical trials. These vaccines exhibit a number of advantages over conventional vaccines that are based on proteins in terms of efficacy, safety, ease of formulation, scale up ability and cost. But there are certain drawbacks associated with nucleic acids.
DNA is a smaller amount immunogenic and it’d integrate in human genome while RNA gets degraded rapidly in about but 10 minutes, but still RNA is found effective for development of vaccines against different diseases.
The efficiency of these vaccines is enhanced by encapsulating in liposomes. Four liposome based vaccines namely: Epaxal, Inflexal, Shingrix and Mosquirix have been commercialized. Lipid nanoparticles became best suited candidate for vaccine formulation because they carry both the antigen and adjuvant efficiently and that they exhibit similar kind of structure and dimensions as that of virus. Apart from incorporating adjuvants, lipid nanoparticles themselves also have been found to exhibit good properties of adjuvant.
For COVID-19, 12 vaccines are approved by FDA and lots of more are under clinical trials. The underlying mechanism in most of these vaccines is to induce neutralizing antibodies against the spike proteins so that human cells prevent its uptake by an angiotensin converting receptor of enzyme (ACE2). There are two approved mRNA based LNPs which incorporates BNT162 AND mRNA-1273.
Pfizer and BioNTech SE has developed LNPs which encapsulate mRNA. They have four candidates (BNT162a1 to BNT162c2). These vaccines are under phase 2 clinical trials with reference number NCT04380701 among healthy individuals of age group 18 to 85 years. With reference number NCT04368728 they are under phase 3 clinical trials. BNT162b2 has shown high efficiency and it’s completed phase 3 clinical test and it’s been approved by FDA for emergency use. Pfizer and BioNTech have been distributing it in USA since December 2020. Another vaccine namely mRNA-1273 encapsulated LNPs has also gained permission for emergency use. Many other lipid nanoparticles based vaccines for COVID-19 are under clinical trials and have high potential for exhibiting efficient response.
![]()

Postgraduate student from NUST Islamabad. I have keen interest in nano-based medicines that have great potential to make world free of deadly viruses like COVID-19.


Very nice effort
Thank you dear
Very informative blog.higly appreciate your work
Good effort.
Thank you soo much
Great👍👍
Very well written…